Why Do Primary Alkyl Halides Undergo Sn2
Primary and secondary alkyl halides can undergo the SN2 mechanism but tertiary alkyl halides react only very slowly. It is possible if we use as a nucleophile a weak base cyanide ion or alcohol.

When Is The Mechanism Sn1 Or Sn2 Chemistry Steps
Steric Hindrance As you add more alkyl groups o the α carbon atom the substrate becomes less susceptible to S_N2 attack A 1 alkyl is sterically unhindered so an S_N2 reaction is likely.
. Do halides undergo sn1. Some of the more common factors include the natures of the substrate carbon skeleton the solvent the leaving group and the nature of the nucleophile. Chemistry questions and answers.
TERTIARY ALKYL HALIDES NEVER SHOW S N 2 REACTIONS. Primary alkyl halides undergo S_N2 mechanisms because a 1 substrates have little steric hindrance to nucleophilic attack and b 1 carbocations are relatively unstable. The carbon is unhindered by bulky groups so the nucelophile can easily attack.
2 More stable carbocations react slower than less stable carbocations. Why do primary alkyl halides undergo SN2 substitution reactions more rapidly. 2009 86 519-524 shows that secondary alkyl halides do not undergo SN1 reactions.
Therefore steric hindrance is one of the major factor in SN2. In SN2 reaction attack of nucleophile takes place from backward direction. Neopentyl halides are not good electrophiles in the way that other primary alkyl halides are primarily due to steric effects but also because of rearrangents and eliminations.
Which of the alkyl halides will undergo SN1 reaction at a faster rate. Since primary alkyl halide is the least sterically hindered among primarysecondary and tertiary alkyl halides. To avoid the loss of HBr.
With polar aprotic solvents primary alkyl halides react faster than sec-ondary halides by the SN2 mechanism whereas tertiary alkyl halides hardly react at all. This reaction does not occur with tertiary alkyl halides because the nucleophilic attack is disabled due to the steric hindrance. Nature of the carbon skeleton.
All the give compounds are tertiary alkyl halides but the bond formed between carbon and iodine C-I bond is the weakest bond due to a large difference in the size of carbon and iodine. This is counter to what we have been and still are in many cases teaching. Whether an alkyl halide will undergo an S N 1 S N 2 E 1 or an E2 reaction depends upon a number of factors.
Whether an alkyl halide will undergo an S N 1 or an S N 2 reaction depends upon a number of factors. But with secondary alkyl halides is more difficult to achieve than with primary. This paper 1 notes in its abstract that As a rule the SN2 reactions of neopentyl bromide and tosylate with sodium alkoxides are extremely retarded by steric hindrance and the yields of alkyl.
3 More stable carbocations react slower than less stable carbocations. The primary alkyl halides are undergoing the S N 2 reaction regardless of solvent. SN1 reactions are preferred when the stability of carbocation is maximum and primary carbocation is less stable.
Murpy TJ J. There are two types of mechanism for alkyl halides SN1 and SN2. Answer 1 of 6.
The SN1 mechanism is a two-stage mechanism where the first stage is the rate determining step. The nature of the nucleophile the solvent and the alkyl halide determine whether nucleophilic substitution takes place by the SN1 or the SN2 mecha-nism. This is counter to what we have been and still are in many cases teaching.
Some of the more common factors include the natures of the carbon skeleton the solvent the leaving group and the nature of the nucleophile. In SN2 mechanism nucleophile attacks the alkyl halide from behind forming an intermediate so there should be less steric hindrance in. 1 Why do primary alkyl halides typically undergo SN2 substitution reactions more rapidly than do secondary or tertiary alkyl halides.
2 Why do primary alkyl halides typically undergo SN2 substitution reactions more rapidly than do secondary or tertiary alkyl halides. So CH33CI gives SN1 reaction most readily. Only those molecules that form extremely stable cations undergo S N 1.
Also asked why do primary alkyl halides undergo sn2. Why do tertiary alkyl halides typically undergo SN1 substitution reactions more rapidly than do secondary or primary alkyl halides.

Solved 2 Why Do Primary Alkyl Halides Typically Undergo Sn2 Chegg Com

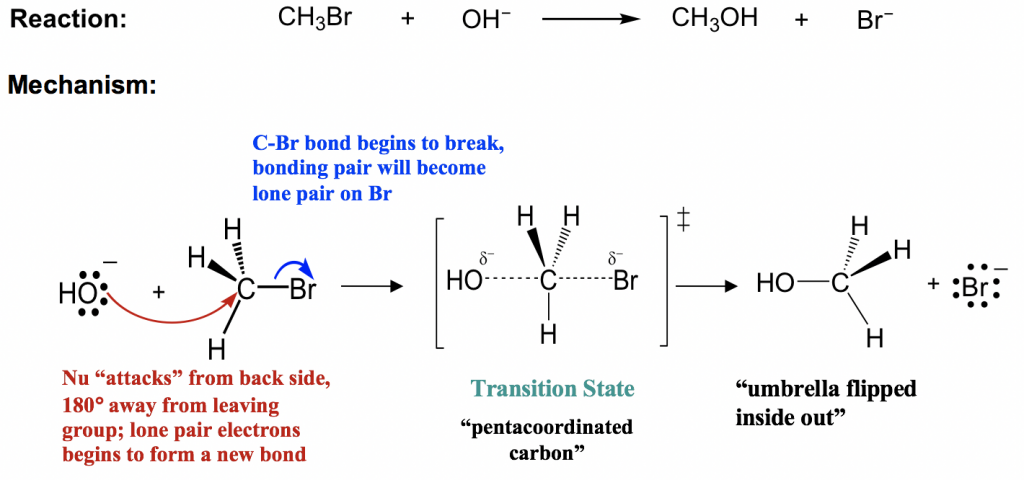
7 2 Sn2 Reaction Mechanism Energy Diagram And Stereochemistry Organic Chemistry I
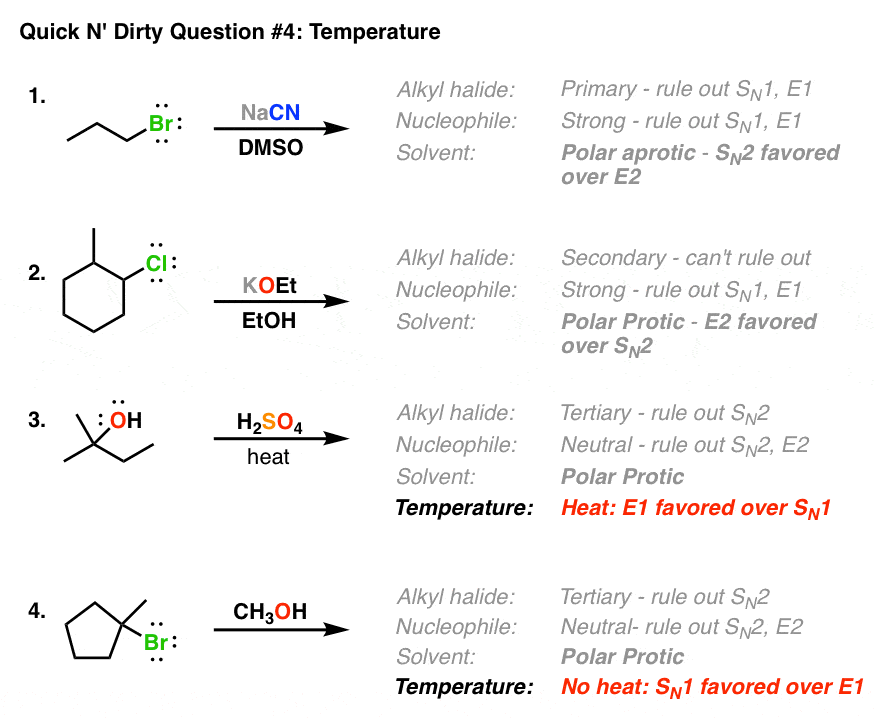
Deciding Sn1 Sn2 E1 E2 4 The Temperature Master Organic Chemistry
No comments for "Why Do Primary Alkyl Halides Undergo Sn2"
Post a Comment